| 2 Research | |
| Section Intro | ab initio | loop modeling | Homology modeling | Energy strain | Domain motion |
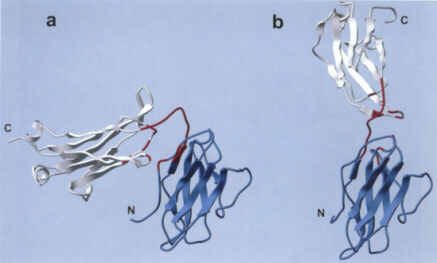 |
| Two crystal forms of Bence-Jones protein (2bjl): (a)BJ closed form; (b) BJ open form. Two subdomains are colored in blue and white |
A method for modeling large-scale rearrangements of protein domains connected by a single- or a double-stranded linker is proposed. Multidomain proteins may undergo substantial domain displacements while their intra-domain structure remains essentially unchanged. The method allows automatic identification of an inter-domain linker and builds an all-atom model of a protein structure in internal coordinates. Torsion angles belonging to the inter-domain linkers and side-chains potentially able to form domain interfaces are set free while all remaining torsions, bond lengths and bond angles are fixed. Large-scale sampling of the reduced torsion conformational subspace is effected with the "biased probability Monte Carlo-minimization" method.
Solvation and side-chain entropic contributions are added to the energy function. A special procedure has been developed to generate concerted deformations of a double-stranded inter-domain linker in such a way that the polypeptide chain continuity is preserved. The method was tested on Bence-Jones protein with a single-stranded linker and lysine/arginine/ornithine-binding (LAO) protein with a double-stranded linker. For each protein, structurally diverse low energy conformations with ideal covalent geometry were generated, and an overlap between two sets of conformations generated starting from the crystallographically determined "closed" and "open" forms was found. One of the low energy conformations generated in a run starting from the LAO "closed" form was only 2.2 A away from the structure of the "open" form. The method can be useful in predicting the scope of possible domain rearrangements of a multidomain protein.
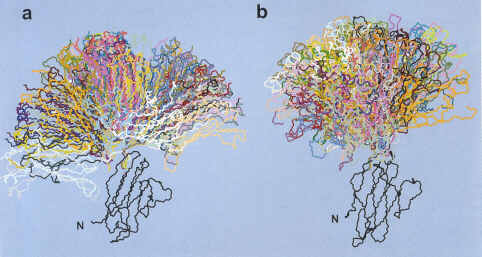 |
| Backbone display of BJ protein conformations generated in the calculations starting from the BJ closed (a) and the BJ open (b) crystal structures. |