2.1 Molecular Modeling
2.1.1 Ab initio peptide structure prediction
| 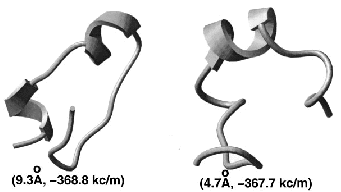 | 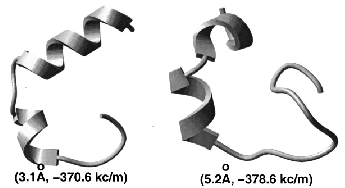 |
Prediction of three-dimensional structures of proteins and peptides by global optimization of the free energy estimate
has been attempted without much success for over thirty years. The key problems were the insufficient accuracy of
the free energy estimate and the giant size of the conformational space. Global optimization of the free energy
function of a peptide in internal coordinate space is a powerful method of structure prediction that out-performs both
Molecular Dynamics, bound by the continuity requirement, and Monte Carlo, bound by the Boltzmann ensemble
gener-ation requirement. We demonstrate that stochastic global optimization algorithms of the first order, i.e., with
local minimization after each iteration (e.g., Monte Carlo-Minimization), have a greater chance of finding the global
minimum after a fixed number of function evaluations. Recently, the principle of optimal bias was mathematically
justified and the Optimal-Bias Monte Carlo-Minimization algorithm (a.k.a. Biased Probability Monte Carlo-minimization)
was successfully applied to theoretical ab initio folding of several peptides, resulting in more than a 10-fold increase in
efficiency compared to the Monte Carlo-Minimization method. The square-root bias is shown to be comparable in
performance with the previously derived linear bias. A 23-residue peptide of beta-beta-alpha structure can be
predicted from any random starting conformation.
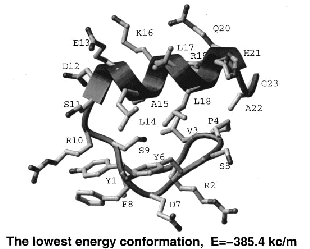
[J.Computational Physics - 1999] [PDF]


