The beauty and the frustration of science are that they are constantly producing surprises. Almost three decades after Christian Anfinsen had won the Nobel Prize for demonstrating that protein folding is governed solely by the protein itself, other scientists discovered that some proteins have helped in the process. This help consists of proteins called chaperones (or chaperonins) that are associated with the target protein during part of its folding process. However, once folding is complete (or even before) the chaperone will leave its current protein molecule and go on to support the folding of another.
Proper folding of some proteins appears to call for not just one chaperone, but several. Especially clear evidence for such multi-step chaperoning is provided by test-tube experiments on a protein known as rhodanese. Proper folding of this protein, the experiments show, requires five different chaperone-type proteins acting at two distinct steps in the operation. Early in the folding process, rhodanese binds to a chaperone known as DnaK; the complex that binds a further chaperone: DnaJ. Somewhat later, a protein known as GrpE catalyzes transfer of the partially folded rhodanese to another chaperone, GroEL, and its partner, GroES. These latter two proteins then see rhodanese all the way through to its properly folded state.
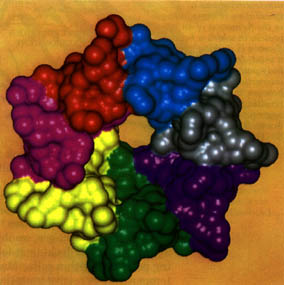
Several lines of evidence suggest that chaperones' primary function may be to prevent aggregation. For example, a chaperone found in the `power plant' organelles of mammalian cells (but otherwise similar to GroEL) has been shown to consist of 14 protein chains arranged as two doughnuts stacked on top of each other (see figure). The chaperoned protein sits inside the two doughnut holes, safely sequestered from other molecules with which it might aggregate.
A role for chaperones in preventing aggregation is also suggested by what happens to mammalian proteins produced in bacteria. Although bacteria have chaperones, they are not the same as those in mammals. It is thus easy to imagine that they may be relatively ineffective toward mammalian proteins, and that this results in the aggregation so often seen. Indeed, there has been one case in which bacteria engineered to overproduce their own chaperones successfully produced a mammalian protein that otherwise irretrievably aggregated. Unfortunately, this approach has failed in other cases. And no one has yet reported introduction of mammalian chaperones into bacteria to help produce soluble mammalian proteins. Yet this, along with the introduction of mutations that block the aggregation pathway and the discovery of small molecules that prevent aggregation, is one of the most promising ways to overcome the roadblocks that biotechnology companies have so often encountered.
 |
The molecular surface of the immunodominant heat-shock chaperonin-10 of mycobacterium leprae shows the multiple subunits of the protein complex forming one doughnut layer (Protein data bank entry 1lep). The second complex would stack together with this complex having the chaperoned protein inside the open hole. Each subunit is colored differently. (Courtesy: Stanley Krystek, Bristol-Myers Squibb, Pharmaceutical Research Institute) |