|
MMTools
Teraflop computing will permit biomolecular simulations of larger
systems and longer time scales, but the increased computing power should
also be invested to improve the analysis of simulations, as well as to
improve their statistical significance. The Resource for Biomolecular
Modelling and Bioinformatics has developed the molecular dynamics
program NAMD2 that takes advantage of massively parallel computers and
it has already gained much experience with teraflop scale simulation
projects. The Resource wants to develop now for such projects a library
of Molecular Modelling Tools, MMTools, that monitor physical
characteristics of simulated systems, and analyze them in the framework
of advanced statistical mechanics. MMTools will be an extendable library
of modular routines (written in C++, like NAMD2), which can be invoked
by the user through Tcl scripts. The user will have the option to (1)
analyze and process on-the-fly the relevant data of a molecular
dynamics run, by invoking MMTools via the Tcl scripting capability of
NAMD2, or (2) use MMTools to process and analyze completed molecular
dynamics simulations. An electronic textbook will be provided that
explains the concepts and specific tools as well as illustrates MMTools
through examples.
1 Introduction
The Resource for Biomolecular Modeling and Bioinformatics proposes to
establish a library of analysis and data processing tools, hereafter
referred to as Molecular Modelling Tools (MMTools), for molecular
dynamics (MD) simulations. MMTools will be needed because of the
increased scope of MD simulations in the era of teraflop computing. Such
simulations will cover orders of magnitude longer times and larger system
sizes, and permit better sampling; this provides an opportunity
to improve the statistical significance of simulations. The power of
teraflop machines can also be used to calculate a more extensive set of
physical characteristics for a simulated system.
MMTools will consist of an extendable set of modular
routines, written in C++. The routines will be accessible via a standard
Tcl command language script, provided by the user. A Tcl interface will
link MMTools to the molecular dynamics program NAMD21 developed at the
Resource, which will permit the user to invoke MMTools during an NAMD2 session for
on-the-fly monitoring and analysis. Through Tcl scripts, the user will
also have the option to employ MMTools for processing MD data of
simulations completed with NAMD2 or any other MD
program.
Initially, MMTools will comprise the following ten tools: (1)
correlation functions - for calculating equal-time-, auto- and
cross-correlation functions; (2) principal component analysis
- for identifying and characterizing slow modes in proteins; (3)
linear response theory - for investigating the response of a
biomolecular system to an external weak perturbation; (4)
hysteresis - for analyzing the nonlinear and time-delayed
response of a system to a periodical external perturbation; (5)
echoes - for studying the coherent excitations of protein
modes via consecutive sudden perturbations; (6) time series
analysis - for reaction coordinate analysis and for reconstruction
of potential of mean force (PMF); (7) mean first passage time
analysis - for describing potential barrier crossing events; (8)
spin-boson model - for modeling spectral line shapes and
(potential) curve crossing phenomena in proteins; (9) vector
quantization of protein dynamics - for determining topologically
discrete representations of manifolds covered by protein dynamics; and
(10) a test suite - for testing the functionality and
performance of the currently available MMTools for three sample systems
of different size, i.e., a small protein (1000 atoms), a medium
protein-water system (10,000 atoms), and a large protein-lipid-water
complex (100,000 atoms).
As already mentioned, MMTools can be readily linked (via Tcl) by the
user to an NAMD2 run. Moreover, the user can check the sampling of data
during runtime using the BioCoRE2 monitoring tools
provided for NAMD2. At certain intervals the tools carry through the
computational analysis in terms of physical characteristics like
correlation functions, response functions, running averages of physical
observables, etc., providing a run time view of the various
characteristics.
The analysis is based to a large degree on concepts from non-equilibrium
statistical mechanics that are conceptually and mathematically demanding
and unfamiliar to many investigators. For this reason a detailed
tutorial in the form of an electronic textbook will be developed that
explains the physical theory behind MMTools and illustrates its use
through examples.
MMTools will be distributed along with all software of the Resource
through the web3. The use of MMTools will be
evaluated both for projects carried out at the Resource and for user
projects. Workshops on the use of Resource software will include
introductions into the use of MMTools.
2 Background and Significance
Molecular dynamics simulations and molecular modeling
are today essential research instruments in biomedicine that complement
observation, permit rational approaches in design of experiments,
provide access to complex data and models, and yield atomic level
understanding of cellular processes. The development of these
instruments were closely tied to advances in computing technologies,
starting with the DEC 780 providing main frame computer power in the
molecular biology laboratory to the recent Cray T3E that permits
routine nanosecond simulations of fully solvated proteins.
Presently, technology promises another great advance in computing power
towards teraflop and even petaflop speeds employing very large
parallel machines. On this new generation of computers, simulations can
be carried out for systems thousand times larger or over time scales
thousand times longer than previously, permitting the study of
protein-protein and protein-nucleic acid recognition and assembly, the
investigation of integral functional units of cells, e.g., eventually a
complete ribosome, and bridging the gap between computationally feasible
and functionally relevant time scales, e.g., for protein folding.
One hopes that the advances through teraflop and petaflop computing will
also improve the quality of molecular modeling through the better
statistics stemming from larger system size and longer time scales as
well as through increasing, if necessary thousand fold, the sample size
of simulations where previously conclusions were based often on single
simulation runs. One also hopes that the increased computer power will
serve to monitor simulations better, collecting in the course of runs
all key physical attributes that pertain to biological function. But to
realize these hopes requires actually a considerable investment in
program development that is beyond the capacity of most biomedical
researchers involved in molecular modeling. The challenge is
particularly great in the present case since the needed tools stem from
the field of non-equilibrium statistical mechanics, a rather advanced
subject that is not taught in most curricula.
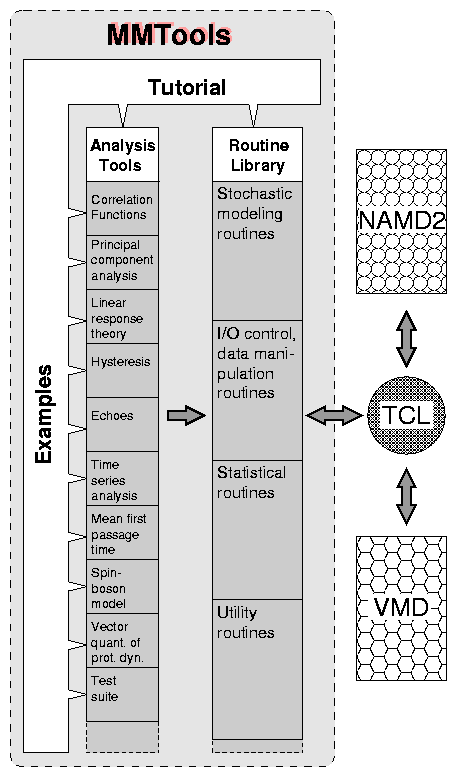
|
Figure 1: Schematic structure of MMTools. The three main components
of MMTools are (i) a collection of Analysis Tools, (ii) a Routine
Library, and (iii) a Tutorial with Examples on how to use MMTools. A
Tcl interface will allow MMTools to communicate with the other
molecular modeling software of the Resource, namely NAMD2 and VMD.
The Resource is in an excellent position to contribute to the
development of the needed physical analysis tools, MMTools, and their
employment. As summarized in Fig. 1, three aspects of its past efforts
provide an ideal starting point for MMTools, namely, the architecture of
the Resource's modeling program NAMD2, the expertise from past projects,
and a considerable investment into a tutorial text through the teaching
and dissemination efforts at the Resource. NAMD2 has been designed to
work with Tcl scripts that can control simulations. In the present case
this permits that MMTools is provided in the form of a library of
routines (written in C++) which can then be invoked by NAMD2 via a Tcl
script. During the past decade, the Resource has already developed
tools based on non-equilibrium statistical mechanics for a broad range
of simulation systems and tasks. This experience furnishes now a basis
to turn ad hoc solutions of the past into a library of systematic tools.
Lastly, the Resource has developed a course at the Physics Department of
the University of Illinois at Urbana-Champaign, taught since several
years, that lead to an electronic textbook4 which can now
be readily extended into a tutorial for MMTools.
The need for monitoring and analysis tools are illustrated below for
three examples.
(1) Structure-function relationship of the purple membrane in
Halobacterium salinarium.5 - The system
studied involves a hexagonal, two-dimensional crystalline array that
exists in the cell wall of some halobacteria with a very small lipid
content and a single protein component (bacteriorhodopsin). The purple
membrane absorbs sun light and converts it into a proton-motive force
across the membrane, switching its proton pumping acticity off when the
potential exceeds about 200 mV. The project at the Resource combined
crystallographic data from various groups to develop a complete
structural model of the purple membrane that comprises all protein and
lipid components with few uncertainties [1].
NAMD2 constant pressure (NpT ensemble) simulations with full
electrostatics and periodic boundary conditions for non-orthogonal cells
(23,783 atoms) are presently carried out. In order to explain the purple
membrane's mechanism a key characteristic, the tight electrostatic
coupling between the system's chromophore retinal and the protein, must
be investigated. This requires the calculation and monitoring of (i)
excitation energy time series, (ii) various overall dipole moments,
(iii) correlation and response (e.g., dipolar response) functions, (iv)
spectral line shape functions, (v) specific heats, and (vi)
thermodynamic potentials of internal water molecules to establish the
role of the latter in proton translocation. The MMTools components
needed for this set of analyses are: correlation functions,
linear response theory, hysteresis, time
series analysis.
(2) Atomic force microscopy and steered molecular dynamics
simulations of proteins in the extracellular matrix.6 - Extending a
successful project at the Resource on the molecular mechanism of the
muscle protein titin that involved collaborations with experimental
researchers at U. Washington, Seattle, and Mayo Clinic, Rochester,
the investigators are embarking now on the study of fibronectin and
proteins of the immunoglobulin superfamily that interact with integrins
and trigger cellular responses in the extracellular matrix[2].
The simulations need to relate protein architecture and mechanical
properties controlled through side group packing, hydrophobic core
interactions and hydrogen bonding to mechanical properties that protect
proteins from unfolding and provide tensil sensory action, e.g., through
the RGD loop in fibronectin. The simulations need to monitor time
series of conformational and energetic properties as well as of applied
forces; an analysis of these data should yield correlation and response
functions, thermodynamic potentials and other properties. Needed MMTools
components are: time series analysis, mean first
passage time analysis, correlation functions,
hysteresis.
(3) Ion selectivity of the KscA potassium
channel.7 - The
potassium channel KscA in Streptomyces lividans exhibits high
selectivity for potassium over sodium ions while maintaining very high
conductivity levels. The channel, recently structurally resolved, is
found to be closely related to potassium channels in cells of many
species and, hence, an explanation of its selectivity and control is
highly desirable. A project at the Resource has established a model of
the channel in an intact PEPG bilayer-water system using NAMD2 in NpT
ensemble mode with periodic boundary conditions and full electrostatics.
The project involved steered molecular dynamics, applying external
forces in order to accelerate the conduction process. This technique
requires monitoring of the time series of the applied force and ion
velocity as well as position in order to abstract the potential of mean
force governing conduction. The method permits studying the system
dynamics actually in a higher dimensional setting that accounts for the
concertedness of ion, water and protein motion. The method corresponds
closely to the WHAM approach to umbrella sampling, except that it
permits the analysis of non-equilibrium situations [3]. The
required components of MMTools are: time series analysis,
correlation functions, principal component analysis,
mean first passage time analysis.
These projects and others at the Resource illuminate essential needs of
monitoring and analysis tools. The following list summarizes tools that
are lacking today in many cases and that biomedical researchers require
for the type of projects described. References provided below
demonstrate the previous efforts of the Resource, but furnish
information on the important contributions of other researchers only
indirectly through the citations found in the given references.
Correlation functions, e.g., velocity correlation functions
[4] or energy correlation functions, characterize dissipative
processes or redox processes [5,6,7]; position and
force correlation functions yield information which identifies soft
modes of proteins [8].
Principal Component Analysis describes major motions of
proteins that might be functionally relevant, e.g., fluctuations
describing gate opening and closing in ion channels. Large scale
motions of proteins are typically not revealed in crystallographic
structures, but might be deducible from them through long time
simulations when properly analyzed [8]. Algorithms are
usually based on diagonalizing position or force correlation functions,
but there exist also methods that permit the determination of large
amplitude motions without sampling and diagonalizing correlation
matrices beforehand, rather gathering information on-the-fly
[9,10].
Linear response theory. The fluctuation-dissipation theorem
(FDT), that holds in the limit of linear response theory, states a
relationship between many correlation and response functions. This
relationship can be exploited to either avoid the calculation of
response functions (that require perturbations in separate simulations)
or to provide a consistency check of simulation runs when both
correlation and response functions have been gathered. The
FDT has been successfully applied in several instances, e.g., in the
study of electron transfer in proteins (e.g., photosynthetic reaction
center [5,6]), or the response of a protein to external
electric fields[11].
Hysteresis. Hysteresis is a phenomenon that accompanies all
cyclic processes in proteins, but can also serve as a probe when cyclic
conditions are artificially enforced, e.g., through oscillatory fields
or periodic forces in atomic force microscopy. The phenomenon reveals
basic physical properties, e.g., frictional elements, as well as
non-linear couplings underlying a process. A systematic theory has been
developed only recently, the Resource having contributed both
conceptually and technically [12,11].
Echoes. Velocity replacement [13] and temperature
quench [14] echoes probe the coherent motion in proteins with
phase relaxation times of about 1 ps. The methodology is based on a
set-up that can only be realized numerically, limiting somewhat the
relevance of the approach. However, echoes provide presently the only
model-free method to address the long-standing problem of coherent
vibrations in proteins [15].
Time series analysis. Steered molecular dynamics simulations
manipulate biomolecular systems through the application of
(multi-dimensional) forces F(t) that lead to responses as
measured, e.g., through conformational transitions captured by
x(t)
[16,17,18,19,20,21,22,23,24,25,2].
Analysis of the resulting time series {F(t),
x(t),v(t)}, corresponding to the manipulated
biomolecular
system, in terms of stochastic models can yield certain attributes,
e.g., thermodynamic potentials, potentials of mean force (PMF), binding
affinities, or reaction barriers [26,3,27]. A most
important application is reaction coordinate analysis: in this case
steered MD simulations select either intuitively or by some systematic
method a reaction coordinate for a process of interest; the system is
then ``pulled'' along this (possibly multi-dimensional) reaction
coordinate; the behavior of the system is finally monitored and analyzed
through the time series analysis method. The desired results are the
reaction coordinate, diffusion coefficient D and friction coefficient
g along the coordinate, as well as the PMF [26,3].
Mean First Passage Time Analysis. This method combines several
trajectories from steered molecular dynamics simulations governing a
barrier crossing or other events characterized through first passage
times[27]. The distribution of passage times monitored, in
combination with a stochastic model, permits one to deduce model
characteristics, e.g., height and shape of reaction barriers.
Spin-boson model. The spin boson model describes the coupling
of a two-state quantum system, as encountered for example in an optical
transition or in a redox process, to a protein described as a collection
of harmonic oscillators[28,7,29,30]. The characteristics of
the coupling can be derived from correlation function properties and
certain distributions. The spin-boson-model, widely applied in physical
and biological theory, furnishes then the spectral line shape function
and its temperature dependence and, hence, rates of optical or electron
transfer events (assuming that the electron coupling is known).
Vector quantization of protein dynamics. This is an algorithm
for topologically faithful discrete representations of manifolds
[31,32], e.g., those covered by protein dynamics. The
algorithm allows one to sample protein motion and to represent the
result through a discretized space that is optimally matched to the
sample. This permits one to sample the phase space of a protein to
identify reaction paths or conformational distributions. The method
yields also a multi-resolution analysis of protein structures that
allows one to combine crystallographic, electron microscopy, and
modeling data [33].
Test suite. For the testing of molecular dynamics simulations
using widely different simulation schemes a representation in terms of
trajectory data is unsuitable. One rather wishes to represent
simulations through the key physical properties of a modeled system,
e.g., correlation and response functions, as well as, thermodynamic and
relaxation properties. A test suite within MMTools will be developed
covering a small (1000 atoms), a medium (10,000 atoms), and a large
(100,000 atoms) system, as described above. An extensive set of
calculations will be carried out on these systems and thermodynamic data
taken. Future simulation methods can use this test suite to check if
properties are properly reproduced in new types of algorithms and
modeling approaches. The test suite will be also used on other MD
programs, e.g., Charmm8 and Amber9.
3 Preliminary Studies
The Resource has continuously demonstrated the ability to successfully
apply advanced computational technology to a wide range of challenging
problems in structural biology. The Resource has established a
leadership role in large scale simulations, as is evident from the
record of completed and published applications
[34,35,36,37,25]. Resource researchers
invested their efforts in advanced software, novel algorithms and
state-of-the-art hardware. The software developed is freely available
to the biomedical community and is widely distributed.
We have taken a broad approach, reflected in the development of (1)
NAMD2, a parallel, object-oriented molecular dynamics program, designed
for high-performance simulation of large biomolecular systems
[38], (2) VMD10, the Resource's popular
molecular graphics program [39], and (3) BioCoRE, the
Resource's integrated, tool-oriented computing and communication system
to support collaboration in the field of structural biology
[40].
The computational and graphics laboratory of the Resource is unsurpassed
amongst academic biomedical institutions. To a large extent, the
success achieved can be attributed to the multidisciplinary approach
taken by investigators from departments of computer science, physics,
chemistry and biophysics.
The Resource was among the first groups in structural biology to see the
promise of parallel computer architectures
[41,42,43,44,34,45]. These new
architectures require programming techniques and concepts which can be
very difficult to retrofit in code originally meant for serial
execution. We have also implemented several state-of-the-art algorithms
in NAMD2, which are applicable in both serial and parallel computing
environments, and enable users to perform ever-larger and longer
simulations. For example, we incorporated the fast N-body solver
DPMTA [46], making it practical to include full effect of
electrostatics into MD simulations. We also developed and implemented
the multiple time scale integration method Verlet-I [43], also
known as reversible RESPA [47], which has greatly improved
simulation performance. NAMD2 has been designed as a flexible
program, incorporating many options to control integration methods,
force field parameters, etc. NAMD2 is user friendly and is easy to
modify and extend to serve goals we have not foreseen. This
modifiability is accomplished in NAMD2 through extensive documentation
at both user and programmer levels, and free availability of source code
written with an object-oriented design. NAMD2 is unique in the extent
to which other researchers can transform it to perform specific tasks.
Via its Tcl scripting language capability NAMD2 can easily be
interconnected with other Tcl based client applications. MMTools
will exploit this unique feature of NAMD2 providing, thereby, on-the-fly
data analysis and processing capabilities.
VMD is a mature visualization environment for structural biology.
Many research groups have adopted VMD for plain visualization, for
docking studies, structure refinement, trajectory analysis, and
interactive dynamics [48]. Recently added capabilities include
enhanced flexibility in display options, extensions to the scripting
language, static docking, and structure alignment. Through the enhanced
scripting language, new analysis routines may now be written without
recompilation of VMD. The improved scripting language allows nearly
all mouse actions to be logged to a script file, edited, and replayed,
making it easy to produce tutorials or high-quality movies. VMD runs
on all major platforms, including Windows.
In supporting their various projects, researchers at the Resource have
made fundamental contributions to the physical analysis of MD
simulations.
Resource researchers had pioneered the use of the correlation function
methods to study relaxation processes in biopolymers, such as proteins.
Already twenty years ago, they used the velocity auto-correlation
function in studies aimed to demonstrate that MD simulations, and their
stochastic extensions, provide a consistent picture of protein dynamics
over a wide range of time scales [4]. Other relevant, and
very important, contributions of the Resource researchers include the
following: the theory of first passage times [49,50] and
general moment expansion [51] to describe molecular relaxation
processes; the theory of spin chemistry and magnetic field effects that
had been based on new theories of stochastic quantum mechanics
[52,53]; the development and use of stochastic quantum
dynamics for redox processes, in particular, the spin-boson approach
[28,7,29]; the development of the theory of hysteresis
[12,11]; the development of time series analysis
generalizing umbrella sampling approaches to non-equilibrium
manipulations [26,3]; the development of some of the first
neural network algorithms, e.g., for principle component analysis
[9,10] and for the representation of complex manifolds
[54,32]; and, finally, contributions to the principle
component analysis of MD simulations through its investigation of the
sampling issues involved [8].
4 Research Design and Methods
In general, MD programs offer very limited analysis capability for the
simulated data, perhaps because it is assumed that the user will be able
to determine and reconstruct various quantities and properties of
interest of the simulated system from the saved (position and velocity)
trajectory files, and energy and temperature outputs. However, in the
age of teraflop and petaflop computing the size of the simulated
biomolecular systems, as well as, the length of the simulations will
gradually become so large that saving complete trajectory files will
require prohibitively large processing and analysis time, and disk space
for storage. Moreover, since the main goal of MD simulations of
biopolymers, with complex dynamics that span a wide range of time
scales, is to understand their functions, one needs a systematic and
extendable set of analysis tools. Currently such analysis tools are
lacking in a readily accessible library format in the biomedical
community, and MMTools is intended to fill this gap.
4.1 MMTools Overview
As shown in Fig. 1, MMTools will have three interconnected
components: (1) the Analysis Tools, (2) a Routine Library, and (3) a
Tutorial with Examples. Here, we present a brief overview of these
components, while further details will be provided in the following
subsections.
(1) Analysis Tools - represent the core of MMTools, and their
functions have already been described in
Secs. I,II. Each tool will consist of
a package of complex, interrelated procedures, designed to address a
specific class of problems in molecular modeling. The design and
development of the algorithms on which these procedures are based, will
represent a key part in the development of MMTools. The procedures will
be written in C++ and will be implemented as Tcl commands via the
extension mechanism of the latter. Thus, well defined input, output and
control parameters will make these procedures flexible to be invoked
either directly, as stand alone commands in a Tcl interpretor/script, or
by inclusion into a user provided code.
(2) Routine Library - will consist of a collection of
specialized subroutines and functions (Sec. IV B), which
will be used by the more complex Analysis Tools. Initially, the Routine
Library will comprise four classes of routines (see Fig. 1)
intended for (a) stochastic modeling, (b) input/output control and data
manipulation, (c) statistical data analysis, and (d) useful numerical
calculations (e.g., numerical integration and derivation). These
routines and functions will constitute the building blocks of the more
complex procedures in the Analysis Tools.
(3) Tutorial with Examples - will explain the theoretical
basis of MMTools, and will provide guidance on how to use these
tools via suitably chosen examples (IV C). The tutorial
will consist of an electronic book, distributed via the WWW. Each
component of MMTools will be described in a separate chapter, starting
with a presentation of the physical principles on which the component is
based, followed by the description of the algorithms, implementation
and invocation of the tool. Finally, the usage and features of the tools
will be illustrated by applying them to concrete examples selected from
published and ongoing research work performed at the Resource.
MMTools will be designed to take advantage of the Tcl interface built into
both NAMD2 and VMD. This will make possible the communication and
interaction between these programs and MMTools, allowing for
on-the-fly analysis of the MD simulation results, and for a certain
degree of control of an NAMD2 session via MMTools.
4.2 MMTools Routine Library
MMTools will be built on a set of specialized subroutines and
functions which initially will be grouped according to their purpose and
functionality in the following four groups.
(a) Stochastic modeling routines - will be used for stochastic
modeling and analysis of biomolecular systems studied via MD simulation.
The need for such a package of routines and their importance can be
easily understood by recalling the fact that in most cases, the analysis
of the MD simulation of a biomolecular system focuses usually on small
subsets of relevant degrees of freedom, while the rest of the system is
treated as a stochastic environment. Then, the selected subsystem is
described by a suitable stochastic model, the dynamics of which is
governed by a stochastic differential equation, and the equation of
motion for the corresponding probability density (i.e., the
Fokker-Planck equation). In general, the action of the stochastic
environment on the studied subsystem can be described by an effective
potential of mean force (PMF), and a stochastic Langevin force. The
evaluation of PMF using MD simulation results represents a challenge
for the molecular modeling community. There are several stochastic
processes which can be used for modeling the dynamics of selected parts
of a biomolecule. For example, the Wiener process describes free
Brownian motion, while the Ornstein-Uhlenbeck process is an extension
of the Wiener process in the presence of a linear drift term. Similarly,
Kramers equation, a special form of the Fokker-Planck equation,
represents the equation of motion of the position and velocity dependent
probability distribution function in the presence of an external
potential. In the large friction limit, applicable to most biomolecular processes, Kramers equation becomes the somewhat simpler Smoluchowski
equation.
The set of stochastic modeling routines will contain the following:
- Stochastic differential equation solver for user specified initial
and boundary conditions, and for stochastic Langevin forces drawn either
from several most commonly used distributions (e.g., Gaussian white
noise) or from the data collected via single or multiple MD sessions.
- Fokker-Planck equation solver for user-specified initial and
boundary conditions, diffusion matrix and drift vector.
- Kramers and Smoluchowski equation solver for user specified initial
and boundary conditions, and parameters (friction coefficient,
external force or potential, etc.)
- Discrete and continuous master equation solver for jump processes.
(b) I/O control, and data manipulation routines - will control
the data and command transfer to and from NAMD2 via a Tcl interface.
The researcher will have the possibility to instruct NAMD2 on how and
when to collect and output the data after a specified number of MD
simulation time steps. For example, let us assume that the researcher
wants to study and record the time evolution of the configuration energy
along a certain reaction pathway. To this end, the researcher must
instruct NAMD2 for a simulation underway when to start calculating the
configuration energy, which set of atoms should be selected for this
purpose, which formula should be used for the calculation, with what
frequency should the data be calculated, in which file, in what format
and with what frequency should the data be written on the hard disk,
and, finally, under what conditions should this operation be terminated.
All these tasks will be accomplished by passing a proper Tcl script
(containing commands from this set of routines) to the Tcl interpretor
built into NAMD2.
Another important application of the I/O control routines will be to
assist the researcher in setting up, and automatically launching,
multiple MD simulation sessions for better sampling. For example, once
the system reaches thermal equilibrium, the researcher, by invoking a
Tcl command, can automatically launch new NAMD2 sessions with initial
configurations provided by the running simulation at predetermined
instants of time. The individual simulations can then be monitored via
Tcl commands launched simultaneously with the corresponding NAMD2 sessions
by the same original Tcl command. Such an approach will increase
dramatically the data collection and analysis of multiple MD
simulations, resulting in much better statistics of the collected MD
data.
Under normal conditions, NAMD2, like many other MD programs, outputs the
results of the simulation in DCD trajectory files. A post-simulation
analysis of the coordinate and/or velocity DCD files requires a DCD
reader/decoder. The data manipulation library will contain such
reader/decoder with very useful options, e.g., allowing the researcher
to extract, on the fly, from the full trajectory file only a subset of
the data, by specifying certain groups of atoms, or number of time
frames to be skipped.
A subclass of I/O control routines will be designed to interface
MMTools with the Tcl interpreter built in VMD. For researchers, the
ability of visualizing in real time those aspects of an MD simulation
they are interested in, will enhance significantly the quality of their
MD data analysis. Clearly, the interconnectivity between MMTools, NAMD2
and VMD (see Fig. 1) will provide biomedical researchers with
an extremely powerful and, at the same time, flexible and easy-to-use
molecular modeling environment.
The initial set of I/O control and data manipulation routines will be
continuously extended with new routines and functions in order to
increase the quality and performance of the interconnectivity between
MMTools and NAMD2/VMD.
(c) Statistical routines - will perform basic statistical
processing of the data collected during an MD session, and are required
by all data analysis tools. Collections of functions and subroutines for
numerical statistics are readily available in both commercial (e.g., the
well known NAG and IMSL scientific routine libraries) and freeware
(e.g., the Numerical Recipes [55], and the SLATEC
library [56]) form. Also, the statistics package in
Mathematica [57] provides (via MathLink) a great
collection of statistics functions. Thus, the implementation of this
class of routines will be straightforward, and will provide efficient
functions for calculating very large sets of data: different mean
values, arbitrary moments, variance, standard deviation, covariance,
skewness, kurtosis, correlation coefficient, auto-correlation, different
random number and statistical distribution generators, data filtering,
etc. All these functions will be registered with the Tcl interpreter of
MMTools and will be available as simple commands in any Tcl script.
(d) Utility routines - will be used for different numerical
calculations and will be based on popular freeware libraries for
scientific computing such as Numerical Recipes
[55] and the SLATEC library [56]. Similarly to the
statistical routines, the implementation of these utility programs will
be straightforward, and they will also be accessible to the researcher
as simple Tcl commands. Initially, this class will contain functions and
subroutines for the following: numerical integration and derivation,
direct and inverse FFT, ODE and PDE solvers, Kramers-Kronig transformer,
linear and non-linear curve fitting, linear and non-linear equation
solvers. This class of routines will be extended on a continuous
basis by adding new functions required by any of the MMTools or by any
other program in the Routine Library.
4.3 MMTools Tutorial
The Tutorial for MMTools will have two main components, which will be
distributed via the popular website of the Resource.
The first component of the Tutorial will consist of a Textbook,
available in several different electronic forms, e.g., HTML, PDF, and
PostScript. Both HTML and PDF versions will have hyperlinks for easy
navigation through the text and the examples. The Textbook will have two
parts. The first part will contain a systematic introduction to
stochastic processes with examples from biophysics. The material
presented there will be based on the lecture notes in non-equilibrium
statistical mechanics mentioned in Sec. II.
The second part of the book will contain a thorough description of
MMTools. Each tool will be allocated a separate chapter, which will be
divided into three parts. Part one will describe the rational and need
for the tool, as well as the physical principles, including the relevant
stochastic methods, on which the analysis tool is based. Part two will
describe the structure, design and implementation of the tool. The role
and functions of the individual procedures which build up the tool will
be explained. Finally, part three will provide an example that
illustrates how the tool is to be used.
An appendix will contain technical material such as a description of all
functions and subroutines in the Routine Library, which represent
the building blocks of the more complex procedures in MMTools: a
brief introduction to the Tcl language; a detailed description on how to
invoke MMTools as Tcl commands; and a presentation of the Tcl interface
between MMTools, NAMD2 and VMD.
The second component of the Tutorial will consist of an ``Examples''
book, containing additional examples, exercises and suggested projects,
inspired by completed and ongoing research projects
conducted by Resource researchers. Each example will contain in
electronic form the actual scripts used to apply MMTools in
controlling the simulation, in collecting the relevant data and
performing the desired analysis. The interested user will be able to
reproduce all the results presented in the ``Examples'' book.
4.4 Dissemination and Training
Dissemination and training efforts will be accomplished mainly through
electronic media. The Resource web site will continue to be a key
dissemination and training vehicle. MMTools web pages containing full
source code of freely distributed MMTools, searchable documentation,
on-line tutorials and presentations will be constructed and maintained
by the Resource. Leading federal and academic structural biology web
sites with links to MMTools will establish visibility of the new suite
of MD analysis tools in the biomedical community.
References
- [1]
-
J. Koepke, X. Hu, C. Münke, K. Schulten, and H. Michel,
The crystal structure of the light harvesting complex II
(B800-850) from Rhodospirillum molischianum,
Structure, 4:581-597, 1996.
- [2]
-
P. E. Marszalek, H. Lu, H. Li, M. Carrion-Vazquez, A. F. Oberhauser,
K. Schulten, and J. M. Fernandez,
Mechanical unfolding intermediates in titin modules,
Nature, 402:100-103, 1999.
- [3]
-
J. Gullingsrud, R. Braun, and K. Schulten,
Reconstructing potentials of mean force through time series analysis
of steered molecular dynamics simulations,
J. Comp. Phys., 151:190-211, 1999.
- [4]
-
W. Nadler, A. Brünger, K. Schulten, and M. Karplus,
Molecular and stochastic dynamics of proteins,
Proc. Natl. Acad. Sci. USA, 84:7933-7937, 1987.
- [5]
-
M. Nonella and K. Schulten,
Molecular dynamics simulation of electron transfer in proteins:
Theory and application to QA ® QB transfer in the
photosynthetic reaction center,
J. Phys. Chem., 95:2059-2067, 1991.
- [6]
-
K. Schulten and M. Tesch,
Coupling of protein motion to electron transfer: Molecular dynamics
and stochastic quantum mechanics study of photosynthetic reaction centers,
Chem. Phys., 158:421-446, 1991.
- [7]
-
D. Xu and K. Schulten,
Coupling of protein motion to electron transfer in a photosynthetic
reaction center: Investigating the low temperature behaviour in the framework
of the spin-boson model,
Chem. Phys., 182:91-117, 1994.
- [8]
-
M. A. Balsera, W. Wriggers, Y. Oono, and K. Schulten,
Principal component analysis and long time protein dynamics,
J. Phys. Chem., 100(7):2567-2572, 1996.
- [9]
-
J. Rubner and K. Schulten,
Development of feature detectors by self-organization: A network
model,
Biol. Cybernetics, 62:193-199, 1990.
- [10]
-
J. Rubner, K. Schulten, and P. Tavan,
A self-organizing network for complete feature extraction,
In R. Eckmiller, G. Hartmann, and G. Hauske, editors, Parallel
Processing in Neural Systems and Computers, pages 365-368. Elsevier,
Amsterdam, 1990.
- [11]
-
D. Xu, J. C. Phillips, and K. Schulten,
Protein response to external electric fields: Relaxation, hysteresis
and echo,
J. Phys. Chem., 100:12108-12121, 1996.
- [12]
-
J. Phillips and K. Schulten,
Diffusive hysteresis at high and low driving frequencies,
Phys. Rev. E, 52(3):2473-2477, 1995.
- [13]
-
D. Xu and K. Schulten,
Velocity reassignment echoes in proteins,
J. Chem. Phys., 103:3124-3139, 1995.
- [14]
-
D. Xu, K. Schulten, O. M. Becker, and M. Karplus,
Temperature quench echoes in proteins,
J. Chem. Phys., 103:3112-3123, 1995.
- [15]
-
K. Schulten, H. Lu, and L. Bai,
Probing protein motion through temperature echoes,
In H. Flyvbjerg, J. Hertz, M. H. Jensen, O. G. Mouritsen, and
K. Sneppen, editors, Physics of Biological Systems: From Molecules to
Species, Lecture Notes in Physics, pages 117-152. Springer, 1997.
- [16]
-
S. Izrailev, S. Stepaniants, M. Balsera, Y. Oono, and K. Schulten,
Molecular dynamics study of unbinding of the avidin-biotin complex,
Biophys. J., 72:1568-1581, 1997.
- [17]
-
B. Isralewitz, S. Izrailev, and K. Schulten,
Binding pathway of retinal to bacterio-opsin: A prediction by
molecular dynamics simulations,
Biophys. J., 73:2972-2979, 1997.
- [18]
-
S. Stepaniants, S. Izrailev, and K. Schulten,
Extraction of lipids from phospholipid membranes by steered molecular
dynamics,
J. Mol. Model., 3:473-475, 1997.
- [19]
-
S. Izrailev, S. Stepaniants, B. Isralewitz, D. Kosztin, H. Lu, F. Molnar,
W. Wriggers, and K. Schulten,
Steered molecular dynamics,
In P. Deuflhard, J. Hermans, B. Leimkuhler, A. E. Mark, S. Reich, and
R. D. Skeel, editors, Computational Molecular Dynamics: Challenges,
Methods, Ideas, volume 4 of Lecture Notes in Computational Science and
Engineering, pages 39-65. Springer-Verlag, Berlin, 1998.
- [20]
-
H. Lu, B. Isralewitz, A. Krammer, V. Vogel, and K. Schulten,
Unfolding of titin immunoglobulin domains by steered molecular
dynamics simulation,
Biophys. J., 75:662-671, 1998.
- [21]
-
D. Kosztin, S. Izrailev, and K. Schulten,
Unbinding of retinoic acid from its receptor studied by steered
molecular dynamics,
Biophys. J., 76:188-197, 1999.
- [22]
-
W. Wriggers and K. Schulten,
Investigating a back door mechanism of actin phosphate release by
steered molecular dynamics,
PROTEINS: Struc., Func., and Genetics, 35:262-273, 1999.
- [23]
-
A. Krammer, H. Lu, B. Isralewitz, K. Schulten, and V. Vogel,
Forced unfolding of the fibronectin type III module reveals a
tensile molecular recognition switch,
Proc. Natl. Acad. Sci. USA, 96:1351-1356, 1999.
- [24]
-
H. Lu and K. Schulten,
Steered molecular dynamics simulations of force-induced protein
domain unfolding,
PROTEINS: Struc., Func., and Genetics, 35:453-463, 1999.
- [25]
-
S. Izrailev, A. R. Crofts, E. A. Berry, and K. Schulten,
Steered molecular dynamics simulation of the Rieske subunit motion
in the cytochrome bc1 complex,
Biophys. J., 77:1753-1768, 1999.
- [26]
-
M. Balsera, S. Stepaniants, S. Izrailev, Y. Oono, and K. Schulten,
Reconstructing potential energy functions from simulated
force-induced unbinding processes,
Biophys. J., 73:1281-1287, 1997.
- [27]
-
H. Lu and K. Schulten,
Steered molecular dynamics simulation of conformational changes of
immunoglobulin domain I27 interpret atomic force microscopy observations,
Chem. Phys., 247:141-153, 1999.
- [28]
-
D. Xu and K. Schulten,
Multi-mode coupling of protein motion to electron transfer in the
photosynthetic reaction center: Spin-boson theory based on a classical
molecular dynamics simulation,
In J. Breton and A. Vermeglio, editors, The Photosynthetic
Bacterial Reaction Center: II. Structure, Spectroscopy and Dynamics,
NATO ASI Series A: Life Sciences, pages 301-312. Plenum Press, New
York, 1992.
- [29]
-
K. Schulten,
Curve crossing in a protein: Coupling of the elementary quantum
process to motions of the protein,
In D. Bicout and M. J. Field, editors, Proceedings of the Ecole
de Physique des Houches, pages 85-118, Paris, 1995. Les Editions de
Physique, Springer.
- [30]
- J. Ray and
N. Makri, Short-range coherence in the energy
transfer of photosynthetic light-harvesting systems,
J. Phys. Chem. A, 103(47):9417-9422, 1999.
- [31]
-
T. M. Martinetz, S. G. Berkovich, and K. Schulten,
``Neural gas'' for vector quantization and its application to
time-series prediction,
IEEE Trans. on Neural Networks, 4(4):558-569, 1993.
- [32]
-
T. Martinetz and K. Schulten,
Topology representing networks,
Neural Networks, 7(3):507-522, 1994.
- [33]
-
W. Wriggers, R. A. Milligan, K. Schulten, and J. A. McCammon,
Self-organizing neural networks bridge the biomolecular resolution
gap,
J. Mol. Biol., 284:1247-1254, 1998.
- [34]
-
H. Heller, M. Schaefer, and K. Schulten,
Molecular dynamics simulation of a bilayer of 200 lipids in the gel
and in the liquid crystal-phases,
J. Phys. Chem., 97:8343-8360, 1993.
- [35]
-
K. Schulten, W. Humphrey, I. Logunov, M. Sheves, and D. Xu,
Molecular dynamics studies of bacteriorhodopsin's photocycles,
Israel Journal of Chemistry, 35:447-464, 1995.
- [36]
-
D. Kosztin, T. C. Bishop, and K. Schulten,
Binding of the estrogen receptor to DNA: The role of waters,
Biophys. J., 73:557-570, 1997.
- [37]
-
J. C. Phillips, W. Wriggers, Z. Li, A. Jonas, and K. Schulten,
Predicting the structure of apolipoprotein A-I in reconstituted
high density lipoprotein disks,
Biophys. J., 73:2337-2346, 1997.
- [38]
-
L. Kalé, R. Skeel, M. Bhandarkar, R. Brunner, A. Gursoy, N. Krawetz,
J. Phillips, A. Shinozaki, K. Varadarajan, and K. Schulten,
NAMD2: Greater scalability for parallel molecular dynamics,
J. Comp. Phys., 151:283-312, 1999.
- [39]
-
W. F. Humphrey, A. Dalke, and K. Schulten,
VMD - Visual Molecular Dynamics,
J. Mol. Graphics, 14:33-38, 1996.
- [40]
-
M. Bhandarkar, G. Budescu, W. F. Humphrey, J. A. Izaguirre, S. Izrailev, L. V.
Kalé, D. Kosztin, F. Molnar, J. C. Phillips, and K. Schulten,
BioCoRE: A collaboratory for structural biology,
In A. G. Bruzzone, A. Uchrmacher, and E. H. Page, editors,
Proceedings of the SCS International Conference on Web-Based Modeling and
Simulation, pages 242-251, San Francisco, California, 1999.
- [41]
-
H. Heller, H. Grubmüller, and K. Schulten,
Molecular dynamics simulation on a parallel computer,
Molecular Simulation, 5:133-165, 1990.
- [42]
-
A. Windemuth and K. Schulten,
Molecular dynamics simulation on the Connection Machine,
Molecular Simulation, 5:353-361, 1991.
- [43]
-
H. Grubmüller, H. Heller, A. Windemuth, and K. Schulten,
Generalized Verlet algorithm for efficient molecular dynamics
simulations with long-range interactions,
Molecular Simulation, 6:121-142, 1991.
- [44]
-
J. A. Board, Jr., J. W. Causey, J. F. Leathrum, Jr., A. Windemuth, and
K. Schulten,
Accelerated molecular dynamics simulation with the parallel fast
multipole algorithm,
Chem. Phys. Lett., 198:89-94, 1992.
- [45]
-
M. Nelson, W. Humphrey, A. Gursoy, A. Dalke, L. Kalé, R. Skeel,
K. Schulten, and R. Kufrin,
MDScope - A visual computing environment for structural biology,
Comput. Phys. Commun., 91(1, 2 and 3):111-134, 1995.
- [46]
-
W. Rankin and J. Board,
A portable distributed implementation of the parallel multipole tree
algorithm,
IEEE Symposium on High Performance Distributed Computing, 1995.
- [47]
-
M. Tuckerman, B. J. Berne, and G. J. Martyna,
Reversible multiple time scale molecular dynamics,
J. Chem. Phys., 97(3):1990-2001, 1992.
- [48]
-
J. Leech, J. Prins, and J. Hermans,
SMD: Visual steering of molecular dynamics for protein design,
IEEE Comp. Sci. & Eng., 3(4):38-45, 1996.
- [49]
-
A. Szabo, K. Schulten, and Z. Schulten,
First passage time approach to diffusion controlled reactions,
J. Chem. Phys., 72:4350-4357, 1980.
- [50]
-
K. Schulten, U. Dinur, and B. Honig,
The spectra of carbonium ions, cyanine dyes, and protonated Schiff
base polyenes,
J. Chem. Phys., 73(8):3927-3935, 1980.
- [51]
-
W. Nadler and K. Schulten,
Generalized moment expansion for Brownian relaxation processes,
J. Chem. Phys., 82:151-160, 1985.
- [52]
-
K. Schulten,
Ensemble averaged spin pair dynamics of doublet and triplet
molecules,
J. Chem. Phys., 80:3668-3679, 1984.
- [53]
-
K. Schulten,
The effect of intramolecular paramagnetic-diamagnetic exchange on
the magnetic field effect of radical pair recombination,
J. Chem. Phys., 82:1312-1316, 1985.
- [54]
-
H. Ritter, T. Martinetz, and K. Schulten,
Textbook: Neural Computation and Self-Organizing Maps: An
Introduction,
Addison-Wesley, New York, revised English edition, 1992.
- [55]
-
W. H. Press, S. A. Teukolsky, W. T. Vetterling, and B. P. Flannery,
Numerical Recipes in C,
Cambridge University Press, New York, 2 edition, 1992.
- [56]
-
The SLATEC Common Mathematical Library ((Version 4.1, July 1993) is a
comprehensive software library containing over 1400 general purpose
mathematical and statistical routines written in Fortran 77. It is freely
available on the Internet at the following URL:
http://netlib2.cs.utk.edu/slatec/index.html.
- [57]
-
S. Wolfram,
The Mathematica book,
Wolfram Media, Champaign, IL, 4th ed. edition, 1999.
Footnotes:
1URL:
http://www.ks.uiuc.edu/Research/namd/
2URL:
http://www.ks.uiuc.edu/Research/collaboratory/
3URL:
http://www.ks.uiuc.edu/Development/
4URL:
http://www.ks.uiuc.edu/Services/Class/NSM.pdf
5URL:
http://www.ks.uiuc.edu/Research/newbr
6URL:
http://www.ks.uiuc.edu/Research/smd_imd
7URL:
http://www.ks.uiuc.edu/Research/smd_imd/kcsa/
8URL:
http://master2.lobos.nih.gov/Charmm/
9URL:
http://www.amber.ucsf.edu/amber/amber.html
10URL:
http://www.ks.uiuc.edu/Research/vmd
|