|
Mechanosensitive channels (MS channels) are proteins found in prokaryotic
and eukaryotic cell membranes that open a conductance pore in response
to mechanical stress. MS channels have been implicated in touch, hearing,
cardiovascular regulation, sensing of gravity and osmotic stress. The
bacterial large conductance MS channel (MscL) is thought to play an important
role in rapidly regulating turgor pressure around the cell;
studies have shown that when bacteria are challenged with a rapid osmotic
downshock, many of the smaller cytoplasmic components are jettisoned into the
medium, yet the bacteria remain viable. A permanent leak in the membrane,
however, can eventually kill the bacterium. An understanding of the gating
mechanism of bacterial MscL could thus lead to development of a new class
of antimicrobial agents.
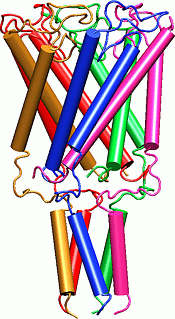 The MscL protein exhibits a high degree of primary sequence conservation within a group of bacteria which includes E. coli, on which most physiology experiments have been performed, as well as M. tuberculosis, from which the crystal structure was obtained. The determination of the crystal structure of MscL (Chang et al., 1998) revealed a protein with a homopentameric structure, approximately 50 Å wide in the plane of the membrane and 85 Å tall. Each 151-residue subunit consists of two transmembrane helices, labeled TM1 and TM2, and a cytoplasmic helix which extends some 35 Å below the membrane. The TMl helices are arranged so as to block diffusion through the channel at their N-terminal ends; this region of the protein also exhibits very high sequence conservation. A loop region between TM1 and TM2 extends into the pore, which may also contribute to the conductance of the channel. Excision of the cytoplasmic domains has been found to have little effect on the gating properties of the channel (Ajouz et al., 2000).
The MscL protein exhibits a high degree of primary sequence conservation within a group of bacteria which includes E. coli, on which most physiology experiments have been performed, as well as M. tuberculosis, from which the crystal structure was obtained. The determination of the crystal structure of MscL (Chang et al., 1998) revealed a protein with a homopentameric structure, approximately 50 Å wide in the plane of the membrane and 85 Å tall. Each 151-residue subunit consists of two transmembrane helices, labeled TM1 and TM2, and a cytoplasmic helix which extends some 35 Å below the membrane. The TMl helices are arranged so as to block diffusion through the channel at their N-terminal ends; this region of the protein also exhibits very high sequence conservation. A loop region between TM1 and TM2 extends into the pore, which may also contribute to the conductance of the channel. Excision of the cytoplasmic domains has been found to have little effect on the gating properties of the channel (Ajouz et al., 2000).
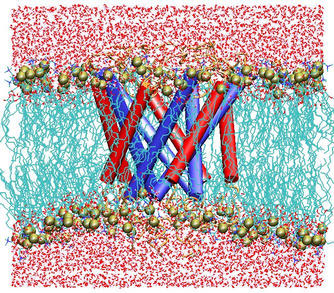 Results of the
simulations of the protein in the full membrane-water system
reflect a protein that is quite stable in the closed state. This is
to be expected from patch-clamp data (Sukharev et al, 1999), which reveal a
channel with zero conductance until significant tension is applied.
Large-scale changes in the shape of the protein could not be expected
during the progress of a 3~ns simulation; it is, therefore, possible
that a much longer simulation could reveal a somewhat different closed state.
We believe that we have described the essential features of this protein
on the time scale of several nanoseconds, and find encouraging correspondence
with experiments.
Results of the
simulations of the protein in the full membrane-water system
reflect a protein that is quite stable in the closed state. This is
to be expected from patch-clamp data (Sukharev et al, 1999), which reveal a
channel with zero conductance until significant tension is applied.
Large-scale changes in the shape of the protein could not be expected
during the progress of a 3~ns simulation; it is, therefore, possible
that a much longer simulation could reveal a somewhat different closed state.
We believe that we have described the essential features of this protein
on the time scale of several nanoseconds, and find encouraging correspondence
with experiments.
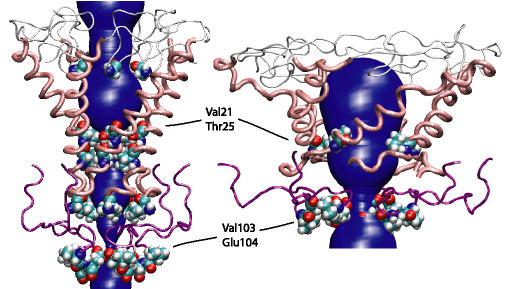
Fluctuations on the scale of individual residues were found to be
in good agreement with corresponding measurements from ESR experiments,
confirming the validity of our protein model. Water penetration in the
pore was found to extend only to hydrophilic residues, i.e., only as far
as Thr25. This result lends support to proposed mechanisms of
MscL gating that postulate a change in the solvent environment of
hydrophobic residues in the pinched region of the protein during gating.
Though a realistic simulation of MscL must include the membrane and
surrounding water, we can investigate the mechanics of the protein
itself without these external media. To this end we conducted a series
of simulations of the same protein structure as in the membrane simulations,
but with no membrane or water present. The simulations were conducted at
constant surface tension and zero normal pressure. To our knowledge, this
is the first time surface tension has been used in molecular dynamics
simulations to elicit a conformational change in a protein.
Our simulations of the bare protein using an applied surface tension
to induce conformational change provided remarkably consistent results:
the protein retained its secondary structure while radically reforming its
tertiary structure to form a large pore. Retention of secondary structure
was an important validity check since the native lipid environment would not
have allowed alternative hydrogen bonds to form. The observation that
the transmembrane helices flattened out corresponds well with recent
measurements made of the effect of membrane thickness on MscL gating
(Kloda and Martinac, 2001).
In these measurements, it was found
that when MscL was placed in a thinner membrane, it remained for a longer
period of time in its open state. This would seem to suggest that the open
conformation of MscL is flatter than the closed structure.
|