|
|
|
|

|

|
The biomedical sciences are enjoying a wealth of data from innovative
new techniques in structure determination, DNA sequencing at the level
of entire genomes, and direct manipulation and observation of single
molecules. The Theoretical Biophysics Group complements these efforts
through the most advanced molecular modeling, bioinformatic, and
computational technologies, extending and refining these technologies
in response to the experimental advances. Our research focuses on
the modeling of large macromolecular systems in realistic
environments. These efforts have produced insight into biomolecular
processes coupled to mechanical force, bioelectronic processes in
metabolism and vision, and the function and mechanism of membrane
proteins. The most recent projects are listed below.
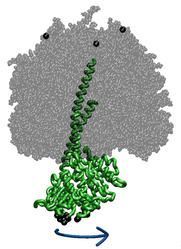 |
ATP synthase is a large multi-protein complex which includes a
transmembrane Fo unit coupled to a solvent-exposed
F1 unit via a central stalk. The stalk rotates within the
surrounding subunits of F1, leading to cyclic
conformational changes in the three catalytic sites in F1
and, thereby, to ATP synthesis. We use steered molecular dynamics to
apply a torque to the central stalk in order to understand the
cooperative interactions that underlie this mechanism. |
| Aquaporins (AQPs) are a large family of membrane proteins
facilitating the transport of water and small neutral soultes across
the cell membrane. They are widely distributed in all life forms,
including bacteria, plants, and mammals, and several pathological
conditions in human are connected to impaired functions of these
channels. Our extensive molecular dynamics simulations on human
aquaporiun-1 (AQP1) and the E. coli glycerol facilitator (GlpF)
have revealed several structural and functional features of these
channels, including the highly conserved secondary structure elements,
the mechanism and energetics of substrate permeation through the
channel, and how they exclude protons while permitting fast water
transport. See more about these channels on our Aquaporin main page.
|
 |
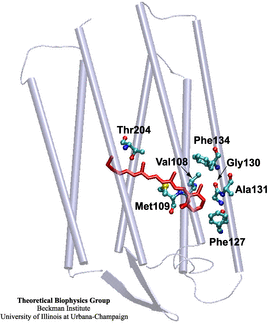 |
The rhodopsin receptors reside in the cell membrane, and
function as sensors of light. These proteins consist of
an apoprotein (opsin) and a retinal chromophore covalently
bound to the apoprotein by a protonated Schiff base linkage
to a lysine residue. While the protonated form of retinal
Schiff base absorbs at about 440 nm in organic solvents, its
maximal absorption is drastically changed after binding to
the apoprotein, an effect known as 'opsin shift'.
A fundamental challenge in vision research has been the
elucidation of the physical mechanisms by which the protein
matrix adjusts the maximal absorption of the chromophore,
using the molecule retinal to detect light at different
wavelengths. The spectral tuning in two
very homologous rhodopsins, sensory rhodopsin II and
bacteriorhodopsin, is investigated by means of a combined ab initio
quantum mechanical/molecular mechanical calculation. |
|
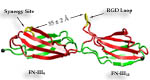
Cells constitute only part of animal tissue, much of tissue is made of
the extracellular matrix (ECM), a flexible mesh composed of several
classes of macromolecule. Fibronectin (FN) is a component of the ECM
that acts as a specific adhesive, forming networks that connect cells
to the giant fibrous molecules that make up the majority of the
ECM. Proper FN fibril formation is required to maintain prop er cell
migration, thus FN plays roles in diseases affecting growth,
development, tumors , and wound healing. We have used SMD simulations
to examine how the mechanical force s present in the ECM affect FN
fibril formation.
|
 |
|
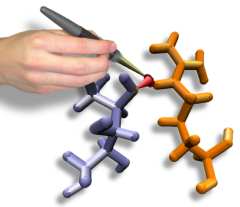
Interactive Molecular Dynamics allows us to pull sugar molecules by
hand through a simulation of the glycerol channel GlpF. As we push
the virtual molecules around, we feel them in our hands as if they
were real. We use this technique to explore features of the channel and
gain new insights into the way it functions. |
 |
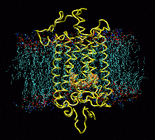 |
Responding to a great variety of ligands including hormones,
neurotransmitters, and peptides, G-protein-coupled receptors (GPCRs)
are the most important drug targets. Yet there is still little known
about the first step of signal transduction. Rhodopsin is a member of
this largest group of transmembrane receptors. The high resolution
structure of rhodopsin determined by Palzcewski et al. (2000) gives us
the opportunity to take a close look at the activation mechanism.
Rhodopsin's ligand, retinal, which is covalently bound to the binding
pocket, absorbs light at 500nm and undergoes
a cis-trans isomerization triggering conformational changes in the
protein. In order to understand the effects of the isomerization on
the protein structure, we are performing molecular dynamics of a
40,000 atom model of rhodopsin in membrane.
|
|
|
|
|