In Exercise Six, the goal is make a comparison between the active sites of the serine proteases, trypsin and subtilisin, and the cysteine protease, papain.
One of the fundamental goals of enzymology is to identify those characteristics of enzyme structure that have led to the unusual levels of rate enhancement that characterize biological catalysts. Frequently, however, catalytic traits are catalogued without any certainty that the behavior under consideration has played an important role in promoting catalysis. It is likely that some aspects of enzyme mechanism merely reflect a historical accident in the ancestry of the enzyme and play no important role in the molecule's ability function (not unlike the vestigial hair on our forearms that offers no particular warmth). Therefore, it is frequently of interest to seek parallels from a variety of enzymes, to show trends and consistent choices that have been made in optimizing catalytic function. For example, if two enzymes perform the same type of chemistry and use the same mechanism, yet they are completely unrelated in other, structural aspects, we would feel comfortable in assuming that the shared mechanism offers some strong advantage to the enzymes.
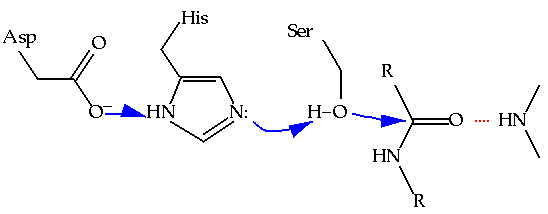
Thus, enzymologists are excited to find examples of convergent evolution: cases where two enzymes of unrelated ancestry have converged on a common catalytic mechanism. An analogous example of convergent evolution in anatomy would be to consider similarities in the morphologies of dolphins and sardines - two unrelated animals that have independently developed similar solutions to swimming. In enzymology, the classic case of convergent evolution is that of the serine proteases. At least twice in history, protein molecules have been adapted to the task of catalyzing the hydrolysis of other proteins through the use of nucleophilic serine residue that is part of a "catalytic triad" including a neighboring histidine and aspartate (Figure 6.1).(1) The trypsin class of serine proteases is structurally distinct from the subtilisin class in every way except in the immediate vicinity of the active site, where the structural details of the catalytic residues are closely shared. The strong analogous nature of these two active site geometries has been taken as clear evidence of the inherently superior qualities of the catalytic triad in hydrolyzing the peptide bond. Armed with such knowledge there have been a variety of attempts to reproduce the catalytic triad in small organic molecules, in hopes of reproducing nature's efficiency in protease activity.(2)

Figure 6.1 The catalytic triad of serine proteases, demonstrating the proton relay involved in increasing the negative charge on the serine oxygen. Another shared feature of the serine proteases is the "oxyanion hole," which helps stabilize the growing negative charge on the peptide oxygen through hydrogen bonding.
With all the interest in the comparisons of trypsin and subtilisin, another related class of proteases is often overlooked. The cysteine proteases are another class of proteolytic enzymes that use nucleophilic catalysis in hydrolyzing the peptide bond, with the significant difference being that they use cysteine instead of serine. Papain is a cysteine protease isolated from papaya fruit which is used as Adolph's meat tenderizer and in Tide. Cysteine 25 has previously been identified as the active site nucleophile. Its function is directly analogous to that of Ser195 in trypsin and chymotrypsin. In this exercise you will further inspect the active site of papain in order to identify other structural features in papain's active site that are analogous to those found in the serine proteases. By adding a third point of comparison, you will further define those structural features that are essential for catalysis, while also being able to distinguish those features that may be essential for enhancing the nucleophilicity of serine from those that are preferable for cysteine.
In order to investigate the binding of peptide substrates to papain, Schroeder and coworkers solved a crystal structure of papain in which the free enzyme has covalently reacted with the inhibitor leupeptin, shown in Figure 6.2.(3) The coordinates for this structure may be found in the 1pop.full. This structure was refined with X-Plor, which includes a number of hydrogen atoms in the structure during refinement. While they are not observed crystallographically, they do appear in the final model. Another unusual feature of this structure is the presence of two types of solvent molecules, HOH and MOH, representing water and methanol respectively. Their presence has no impact on this exercise.
In this structure, cysteine 25 has become covalently attached to a carbonyl carbon through the formation of a thiohemiacetal. Note that this inhibitor is not entirely equivalent to a substrate for the enzyme. The true covalent adduct formed between the substrate and the enzyme would be a thioester with the cysteine sulfur bonded directly to the carbonyl carbon (here a hemithioacetal group). Given this distinction, be careful not to interpret interactions around the carbonyl group too strictly. Differences will exist when the acyl.enzyme covalent complex forms.
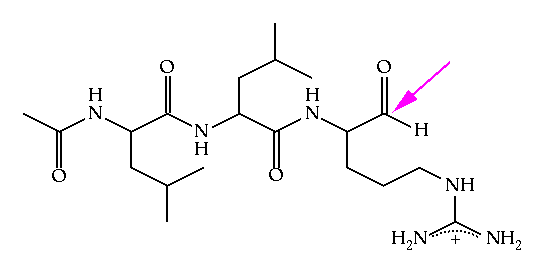
Figure 6.2 Leupeptin, an inhibitor of papain. This molecule is an example of a site-directed covalent inhibitor. The peptide portion of the inhibitor assures that the molecule will bind in the active site, placing the reactive aldehyde functionality (identified with an arrow) in close proximity to the active site nucleophile. In this way, non-selective modification of other nucleophilic residues (particularly lysine) in the protein is avoided.
Inspect the enzyme active site with the bound inhibitor and prepare a figure that clearly demonstrates how the inhibitor is covalently bound to papain. It may be helpful to edit image of the structure to just a small region around Cys 25 by using the following command.
In addition, if the protons in the model distract you, they can be hidden from view with the command:
Examine the region around Cys25. Previous workers have commented on its similarity to that of the serine proteases, especially with regards to the catalytic triad. Investigate the means by which the enzyme activates Cys25 as a nucleophile. Attempt to identify residues that are directly analogous to those in Figure 6.1. Provide appropriate interatomic distances where possible. If differences exist between papain and the serine proteases, offer possible interpretations in the context of cysteine vs. serine activation.
A second facet to catalysis of nucleophilic addition is that the growing negative charge on the carbonyl oxygen must be stabilized in the transition state. How do you think the enzyme performs this task?
1. For a more complete discussion of the active sites of the serine proteases, see Chapter 15 of Branden and Tooze, Introduction to Protein Structure.
2. V. T. D'Souza, K. Hanabusa, T. O'Leary, R. C. Gadwood and M. L. Bender (1985) Biochem. Biophys. Res. Commun. 129, 727-732.
3. E. Schroeder, C. Phillips, E. Garman, K. Harlos and C. Crawford (1993) FEBS Letters 315, 38.
(4) In this structure, the papain chain runs from 1A-212A. The inhibitor comprises residues 300B-303B (the acetyl group is "residue" 300B).