
Crosslink Density of Superabsorbent Polymers
Often viewed as primarily a tool for basic research, synchrotron radiation nonetheless has its share of industrial users looking for solutions to their problems. At the ALS, researchers from The Dow Chemical Company teamed with academic colleagues to conduct x-ray spectromicroscopy studies of superabsorbent polymers (SAPs), materials with a $2 billion annual market for a wide range of products including disposable baby diapers. The experiments were such a success that Dow has been able to use the results to help develop the process technology now being designed for a new SAP-manufacturing plant. Because of this and related work on x-ray spectromicroscopy of polymeric materials, Dow's Analytical Sciences division has conferred on two of the Dow researchers its highest internal award.
|
Superabsorbent polymers are a specific example of a polymer gel. Occurring in both natural and synthetic forms, gels exhibit an intriguing combination of the properties of both liquids and solids. One feature that makes gels useful is their ability to respond strongly to very weak external stimuli, such as minute changes in pH or temperature. For example, a polymer gel might first absorb a quantity of liquid and later release it as the external conditions change. Timed release of pharmaceuticals is one example among many in which a control stimulus determines the rate of release. Crosslinking is a key feature of the polymer microstructure that governs actual performance. Dow sells SAP in the form of small (less than 1.0 mm in size) beads of sodium polyacrylate that is lightly crosslinked to form an insoluble, hydrophilic gel. The ability to soak up great quantities of fluid makes SAPs attractive for use in diapers. But, like a sponge when compressed, some of the fluid is squeezed back out when the baby moves, negating part of the benefit. The strategy for preventing leakage under weight-bearing load is the formation of a thin shell of more tightly crosslinked polymer. The effectiveness of the shell depends in part on the density profile of the crosslinking through the shell, a distance of several microns. Several different methods have been developed to make surface crosslinked SAP gels, but there has been no good way to visualize and assess the resulting core-shell structure and the crosslink density profile. To obtain the desired information the researchers turned to near-edge x-ray absorption fine structure (NEXAFS) spectromicroscopy, using the scanning transmission x-ray microscope (STXM) on Beamline 7.0.1 to make images of the polymers in the fully hydrated state (in excess water). Because the x-ray energy could be tuned to a value where the carbon in the polymer absorbs and the water is almost transparent, they could map the areas where crosslinking was higher by observing the increased carbon content in these regions. |
Baby Diapers Drive Polymer Improvements
|
||
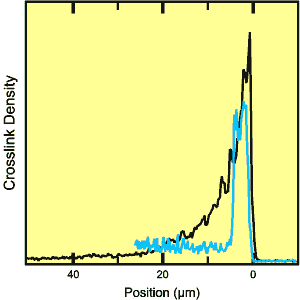
Crosslinking was stimulated by treating the surface of SAP beads with varying amounts of either ethylene glycol diglycidyl ether or glycerol. Sectioned beads were then exposed to 0.9% saline solution to put them in the fully swollen state for imaging. Analysis of the images yielded two extreme cases for the crosslink profile through the shell. In one, the crosslink density decreased smoothly over a distance of 18 microns from a maximum at the outer surface. In the other, the density was uniform over a distance of 5 microns and then dropped abruptly. These differences reflect a complicated interplay between the dynamics of the swelling of the bead in water, the diffusion rate of the crosslinker in the water phase, and the rate of the crosslinking reaction. Dow was able to use this kind of information in designing a new SAP-fabrication plant. 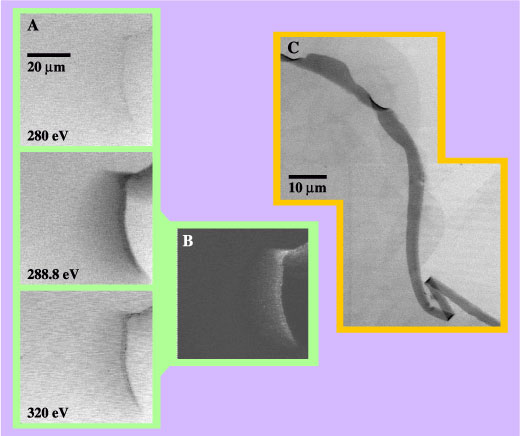
Research conducted by G.E. Mitchell, L.R. Wilson, M.T. Dineen, F. Hayes, and E.G. Rightor (The Dow Chemical Company); S.G. Urquhart and H.W. Ade (North Carolina State University); and A.P. Hitchcock (McMaster University). Research funding: The Dow Chemical Company, National Science Foundation, and Natural Sciences and Engineering Research Council of Canada. Operation of the ALS is supported by the Office of Basic Energy Sciences, U.S. Department of Energy. Publication about this research: G.E Mitchell, L.R. Wilson, M.T. Dineen, S.G. Urquhart, F. Hayes, E.G. Rightor, A.P. Hitchcock, and H.W. Ade, "Quantitative Characterization of Microscopic Variations in the Crosslink Density of Gel" (submitted 2001).
|
|||
More ALS Science