Aquaporin Structure Elucidates Water Transport
From aqueducts to osmosis, water transport is crucial to life. Yet, precisely how life manages the transport of water across membranes has remained a mystery for eons—until now. A team of researchers from the Berkeley Lab Life Sciences Division has solved the structure of aquaporin-1 (AQP1), a membrane protein that controls the movement of water molecules into and out of mammalian cells. It is a member of the aquaporin superfamily, whose members transport water or water and glycerol or urea. The new structure offers a resolution of 2.2 Å, allowing researchers to deduce just how the protein does its job.
|
After preliminary work at the National Synchrotron Light Source
and the Stanford Synchrotron Radiation Laboratory, the team turned
to the Berkeley Center for Structural Biology and ALS Beamline
5.0.2. They began by studying thallium-derivatized crystals of
AQP1 from bovine red blood cells by multiwavelength anomalous
diffraction (MAD). They then refined the resulting data set to
2.2 Å by using crystals grown in the presence of gold cyanide.
The high-resolution structure shows atomic-level details of the
protein and water molecules captured in transit.
|
More Than Just a Bag of Water |
||||||
|
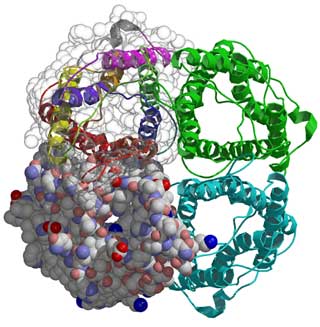
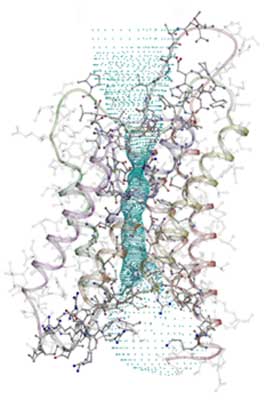
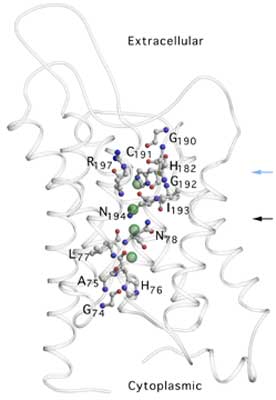
The overall structure of AQP1 is that of a tetramer, the four parts (monomers) of which each define a single pore. These monomers are arranged side by side in a tight cluster, with the pores running parallel. Each monomer in turn comprises six membrane-spanning helices that partially surround two shorter helices. The short non-membrane-spanning helices make up the major portion of the pore. Each pore has a dumbbell-like shape. One broad end is the cytoplasmic vestibule; the other is the extracellular vestibule. The bar of the dumbbell is the selectivity filter, which narrows to a constriction region on the extracellular end. In the new structure, the key elements of the constriction region can be discerned. A series of carbonyl oxygens forms a hydrophilic path across the region and through the rest of the selectivity filter. One of these oxygens, along with a histidine residue and an arginine residue, forms the hydrophilic face of the constriction region. Opposite this face is a hydrophobic face formed by a phenylalanine residue. Three of the four residues that form the constriction region (the arginine, histidine, and phenylalanine residues) are conserved in all known water-specific aquaporins. This observation suggests that the presence of these residues can be used as a marker for identifying other water-specific aquaporins.
An earlier study by a different group—also done at the ALS—revealed the structure of a closely related bacterial channel, the Escherichia coli glycerol facilitator (GlpF), which selectively transports glycerol. The structure found for GlpF differs from that of AQP1 in that its constriction region is about 1 Å wider and slightly less polar. The resulting decreases in steric hindrance (physical blocking) and hydrophilicity favor glycerol transport at the expense of rapid water throughput. Despite the functional difference, the GlpF constriction region is strikingly similar to its AQP1 counterpart. It differs only in the replacement of a histidine by a glycine and a cysteine by a phenylalanine. As both the cysteine and the phenylalanine provide carbonyl oxygens to shape the constriction region, the difference in functionality turns out to depend upon the residue found in the location of the histidine (H182) in AQP1. This residue thus appears to be key in defining selectivity throughout the aquaporin superfamily. Research conducted by H. Sui, B.-G. Han, J.K. Lee, P. Walian, and B.K. Jap (Berkeley Lab). Research funding: National Institutes of Health; U.S. Department of Energy, Office of Health and Environmental Research. Operation of the ALS is supported by the U.S. Department of Energy, Office of Basic Energy Sciences. Publication about this research: H. Sui, B.-G. Han, J.K. Lee, P. Walian, and B.K. Jap, "Structural basis of water-specific transport through the AQP1 water channel," Nature 414, 878 (2001). |
|||||||
More ALS Science