
Spectra of Anionic Fragments Verify Shape Resonances
Shape resonances are a controversial subject among molecular spectroscopists, in part because there has been no agreed-upon way even to identify them when they occur, which is thought to be rather frequently in both gas-phase and adsorbed molecules. So it is good news that an international team at the ALS comprising researchers from the U.S., Italy, Mexico, and Sweden has discovered a method for verifying their presence. The team's approach, demonstrated in carbon monoxide, is to measure the yields of both cation and anion fragments above the molecular photoionization threshold. They showed that, unlike cation spectra, the anion spectra do not contain the shape resonance, whose energy is well known in this molecule. Measuring both therefore pinpointed the shape resonance.
|
As the x-ray photon
energy scans from (say) 20 eV below to 20 eV above the photoionization
threshold for 1s electrons in molecules comprising atoms with low
atomic numbers and p bonding, a progression
of typical spectral features appears. The sequence begins at the
lower energy end with the familiar p*
(Feshbach) resonances that are so useful in molecular fingerprinting
and in calibrating monochromators, progresses through a number of
Rydberg series just below threshold, and ends above threshold with
overlapping features due to doubly excited (two electrons in excited
states) molecules and shape resonances. 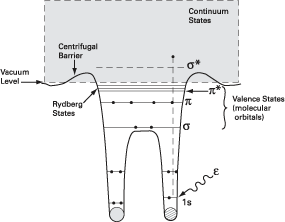
The shape resonance results from a potential barrier that temporarily prevents electrons excited in the resonance energy range from leaving the molecule. Their apparent simplicity, broad energy widths, and well-defined symmetries have made shape resonances a popular object of study in gas-phase molecular science and a common tool for probing orientations and bond lengths in molecules adsorbed on solid substrates. Unfortunately, picking out shape resonances from other spectral features is not so straightforward, thereby raising questions about the validity of parameters extracted from their analysis. For example, peaks due to double excitation can be experimentally indistinguishable from shape resonances. |
Fragmented Molecules Reveal Their Secrets |
||
Against this background, carbon monoxide presents itself as a valuable test bed. It displays a broad peak that is known to be due to a shape resonance and is readily distinguishable from sharper features at somewhat lower energy from doubly excited molecules. Led by researchers from the University of Nevada, Las Vegas, the international team extended the well-known technique of ion-yield spectroscopy to examine negatively charged ion species, as well as the commonly measured positively charged ions, that are created when the molecule breaks into fragments during the decay process following x-ray excitation. The high spectral resolution and signal intensity achieved at ALS Beamline 8.0.1 made anion spectroscopy feasible. 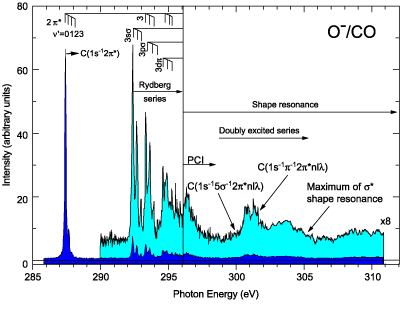
Several pathways exist for creation of ions from simple photoemission, which leaves a CO2+ ion, to photofragmentation, which can result in several cation species and O- as the primary anion. Measuring the O- production as a function of photon energy near the carbon K edge yielded a spectrum containing the various features enumerated above but with no apparent contribution from the known shape resonance. Detailed comparison of cation and anion spectra above the photoionization threshold conclusively demonstrated the absence of the resonance clearly visible in the cation spectra. Anion measurements at the oxygen K edge were only somewhat less conclusive, owing to more overlap with the region of doubly excited states. 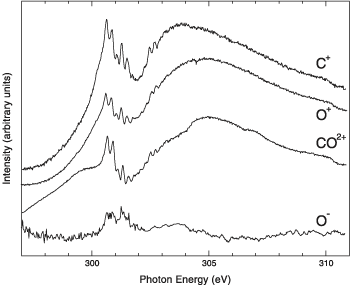
The main message is that shape resonances are completely absent in anion yields. Moreover, there is nothing special about carbon monoxide, so the researchers argue this new approach should apply to many small molecules, thereby providing a new tool to examine core-level resonant processes in general and sort out shape resonances in particular. Research conducted by W.C. Stolte (University of Nevada, Las Vegas, and ALS); D.L. Hansen (Jet Propulsion Laboratory); M.N. Piancastelli (University of Rome, "Tor Vergata," and ALS); I. Dominguez Lopez (Centro Nacional de Metrologia, Mexico); A. Rizvi and A.S. Schlachter (ALS); O. Hemmers and D.W. Lindle (University of Nevada, Las Vegas); H. Wang (Lund University, Sweden); and M.S. Lubell (City College of New York). Research Funding: National Science Foundation; U.S. Department of Energy (DOE), EPSCoR; National Research Council. Operation of the ALS is supported by the U.S. DOE, Office of Basic Energy Sciences. Publication about this research:W.C. Stolte, D.L. Hansen, M.N. Piancastelli, I. Dominguez Lopez, A. Rizvi, O. Hemmers, H. Wang, A.S. Schlachter, M.S. Lubell, and D.W. Lindle, "Anionic Photofragmentation of CO: A Selective Probe of Core-Level Resonances," Phys. Rev. Lett. 86, 4503 (2001). |
|||
More ALS Science