
Gas-Phase Molecules Illuminated from Within
Seeing is believing, and the importance of visualization is obvious at the molecular level, which lies outside the realm of everyday experience. But because traditional techniques for probing within molecules (such as photoelectron diffraction) require knowledge of the molecule's orientation, they can't provide very information-rich pictures for molecules in the gas phase. Addressing this problem, an international collaboration of researchers has demonstrated a multiparticle coincidence technique at the ALS that yields comprehensive photoelectron diffraction data for gaseous carbon monoxide as if the molecules were fixed in space. The most striking aspect of viewing molecules "illuminated" from within this way is how the results reveal, nearly at a glance, the major physical features at play.
|
In x-ray photoelectron diffraction (XPD), a core-level electron is ejected from one atom in a molecule by an incoming x ray. The ejected photoelectron wave, diffracted by neighboring atoms, provides a signature of the nonspherical potential of the molecule. However, to fully observe the rich, three-dimensional structure of the electron wave, knowledge of the orientation of the molecule is required. In most cases, the material under investigation is in solid form (crystal or adsorbate) and can be easily oriented in the laboratory. Determining the orientation of a freely moving molecule, however, requires some experimental finesse.
|
In Living Color |
|
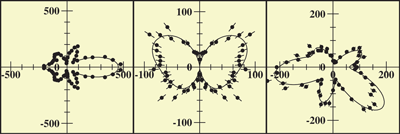
For example, the data can be displayed in a map of the photoelectron momentum vectors, where the azimuthal angle gives the direction of the photoelectron emission, the radial distance gives the photoelectron energy, and the emission intensity is indicated by color. The resulting pictures are rich in physics: one sees the outgoing wave resonating in the molecular potential at a certain energy (i.e. at a constant radius) and displaying interference from reflection off of the oxygen partner ("bright" spot when the molecular axis is aligned with the x-ray polarization e). An alternative depiction, in polar coordinates, lets us compare the experimental photoelectron momentum data to calculations based on linear combinations of partial waves. The high fidelity of the fitted curves to the data demonstrates that the contributions of different partial waves to the photoelectron wave can be successfully extracted from the data. 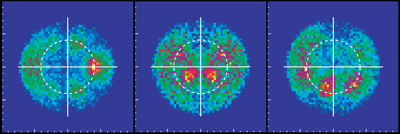 
While the researchers chose a simple ground-state molecule for this demonstration, the technique might also be used to produce time-dependent snapshots of transient species. It may be possible to map the evolution of molecular potentials by initiating a photochemical reaction with a short laser pulse and later probing the molecule using a photoelectron wave generated by a time-delayed x-ray pulse. Thus, this approach to viewing molecules is a major step forward in our ability to obtain comprehensive information about molecular dynamics and structure from photoelectron emission. Research conducted by A. Landers (Western Michigan University); Th. Weber, M. Hattass, O. Jagutzki, A. Nauert, and H. Schmidt-Böcking (Universität Frankfurt); I. Ali, T. Osipov, and C.L. Cocke (Kansas State University); A. Cassimi (Université de Caen); A. Staudte (Universität Frankfurt and Berkeley Lab); M.H. Prior (Berkeley Lab); and R. Dörner (Universität Frankfurt). Research funding: Bundesministerium für Bildung und Forschung; Deutsche Forschungsgemeinschaft; and the U.S. Deptartment of Energy, Office of Basic Energy Sciences (BES), Chemical Sciences, Geosciences and Biosciences Division. Operation of the ALS is supported by BES. Publication about this research: A. Landers, Th. Weber, I. Ali, A. Cassimi, M. Hattass, O. Jagutzki, A. Nauert, T. Osipov, A. Staudte, M.H. Prior, H. Schmidt-Böcking, C.L. Cocke, R. Dörner, "Photoelectron Diffraction Mapping: Molecules Illuminated from Within," Phys. Rev. Lett. 87, 013002 (2001). ALSNews Vol. 189, November 28, 2001
|
||
More ALS Science
